Hospitalized COVID-19 patients at high risk for becoming critically ill and dying had significantly better outcomes if they received the anti-inflammatory drug anakinra researchers have found. Interleukin IL-1 receptor anakinra has been used as an off-label agent for treatment of COVID-19 during the COVID-19 pandemic.

Proposal For The Use Of Anakinra In Acute Respiratory Distress Secondary To Covid 19 Sciencedirect
RECOVERY is a randomised trial investigating whether treatment with Lopinavir-Ritonavir Hydroxychloroquine Corticosteroids Azithromycin Colchicine IV Immunoglobulin children only Convalescent plasma Synthetic neutralizing antibodies REGN-COV2 Tocilizumab Aspirin Baricitinib Infliximab Empagliflozin or Anakinra children only prevents death in patients with COVID-19.
Anakinra covid 19. Your Child and COVID-19. Eg methylprednisolone 1mgkgday IvGTT for 7 days. Furthermore many clinical evidences have indicated the importance of anti-inflammatory therapy in severe COVID-19.
Kineret or anakinra is an interleukin-1. Meanwhile efforts to find an effective treatment to inhibit virus replication mitigate the symptoms increase survival and decrease mortality rate are ongoing. Treatment with anakinra may reduce mortality risk in patients hospitalized with moderate to severe COVID-19 pneumonia according to findings from a systematic review published in Lancet Rheumatology.
Current knowledge on the effect for COVID-19 infection for corticosteroids and anakinra dosing for severe cased with COVID-19 is higher and intravenous. Treatment with anakinra reduces the need for invasive mechanical ventilation and mortality risk in hospitalized nonintubated patients with COVID-19 according to a meta-analysis published in Rheumatology. Treatment with anakinra an interleukin-1 receptor antagonist was found to decrease mortality and reduce the need for invasive mechanical ventilation compared with standard of care SOC in hospitalized patients with COVID-19 according to the results of a meta-analysis published in Infection and Chemotherapy.
Approval 3820 of the National. Swedish Orphan Biovitrum AB Sobi and the Hellenic Institute for the Study of Sepsis have reported positive data from the Phase III SAVE-MORE clinical trial of anakinra plus standard of care SOC in moderate-to-severe Covid-19 pneumonia patients. Further studies are needed to assess the efficacy of anakinra in other selected groups of patients with more severe COVID-19 The CORIMUNO-19 collaborative group 22 January 2021.
The COVID-19 pandemic has led to unprecedented calls for national and now worldwide vaccination evidence tracking via a proposed global vaccine passport. Previous studies have suggested that interleukin IL-1 receptor antagonist anakinra used for the treatment of autoinflammatory disorders may improve hyperinflammatory symptoms in patients. It is thought that this could also help reduce the inflammation and tissue damage associated with COVID-19.
There is an urgent need for effective treatment. The clinical management of COVID-19 has been limited to infection prevention and control measures associated with supportive care such as supplemental oxygen and mechanical ventilation. Sonmez et al We believe that Anakinra may have a role in patients who manifest MAS due to COVID-19 and trials are already underway.
Since March 2020 when the COVID-19 pandemic started in Europe the Hellenic Institute for the Study of Sepsis has launched in Greece the SAVE clinical trial suPAR-guided Anakinra treatment for Validation of the risk and Early management of severe respiratory failure by COVID-19 EudraCT number 2020-001466-11. In this systematic review and meta-analysis researchers aimed to determine the. Hellenic Institute for the Study of Sepsis Phase 2 SAVE-MORE Study Shows Sobis Anakinra Saves Lives of Hospitalized COVID-19 Patients.
Anakinra IV infusion 4 times daily for 15 days. You are encouraged to report negative side effects of prescription drugs to the FDA. Current focus has been on the development of novel therapeutics including antivirals and vaccines.
Hospitalised Covid-19 patients at high risk for becoming critically ill and dying had significantly better outcomes if they received the anti-inflammatory drug anakinra researchers have found. Hospitalized COVID-19 patients at high risk for becoming critically ill and dying had significantly better outcomes if they received the anti-inflammatory drug anakinra researchers have found. Several approaches are currently being used to treat the observed cytokine storm associated with COVID-19 and expectations are especially high for new cytokine-targeted therapies such as tocilizumab anakinra and baricitinib.
Report Problems to the Food and Drug Administration. Like the IL-6 inhibitors the BMC protocol has targeted Anakinra to phase IIb of disease. In November 2019 researchers at the University of Manchester reported that Anakinra might have a use in preventing breast cancer from spreading to the bones.
Anakinra has a shorter half life 3-21h than IL-6 inhibitors. Anakinara is undergoing multiple clinical trials to treat COVID-19 patients by targeting mechanisms in patients with hyperinflammation. Kineret anakinra is a recombinant nonglycosylated form of the human interleukin-1 receptor antagonist IL-1Ra.
However its exact benefits for patients with moderate to severe. Anakinra did not improve outcomes in patients with mild-to-moderate COVID-19 pneumonia. SuPAR-Guided Anakinra Treatment for Management of Severe Respiratory Failure by COVID-19 is a large pivotal confirmatory phase III randomized controlled trial RCT in.
In order to test the drug sold as Kineret by Swedens Sobi Inc the researchers looked for patients with high blood levels of a protein called suPAR. Its active substance anakinra blocks the activity of interleukin 1 a chemical messenger involved in immune processes that lead to inflammation. As of March 12 2020 coronavirus disease 2019 COVID-19 has been confirmed in 125 048 people worldwide carrying a mortality of approximately 371 compared with a mortality rate of less than 1 from influenza.
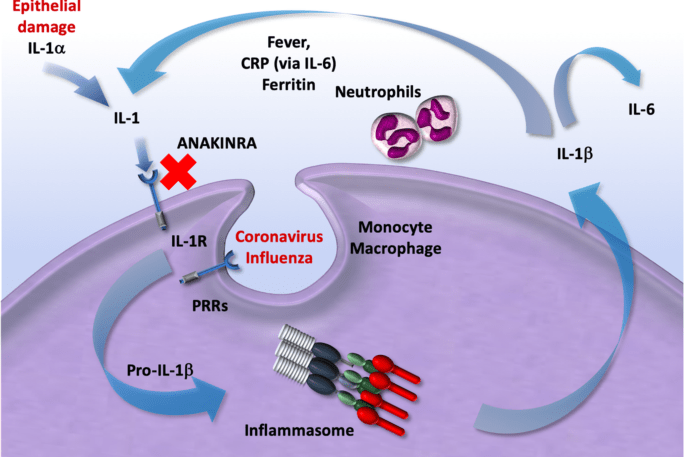
Covid 19 The Role Of Immunomodulators In Treatment
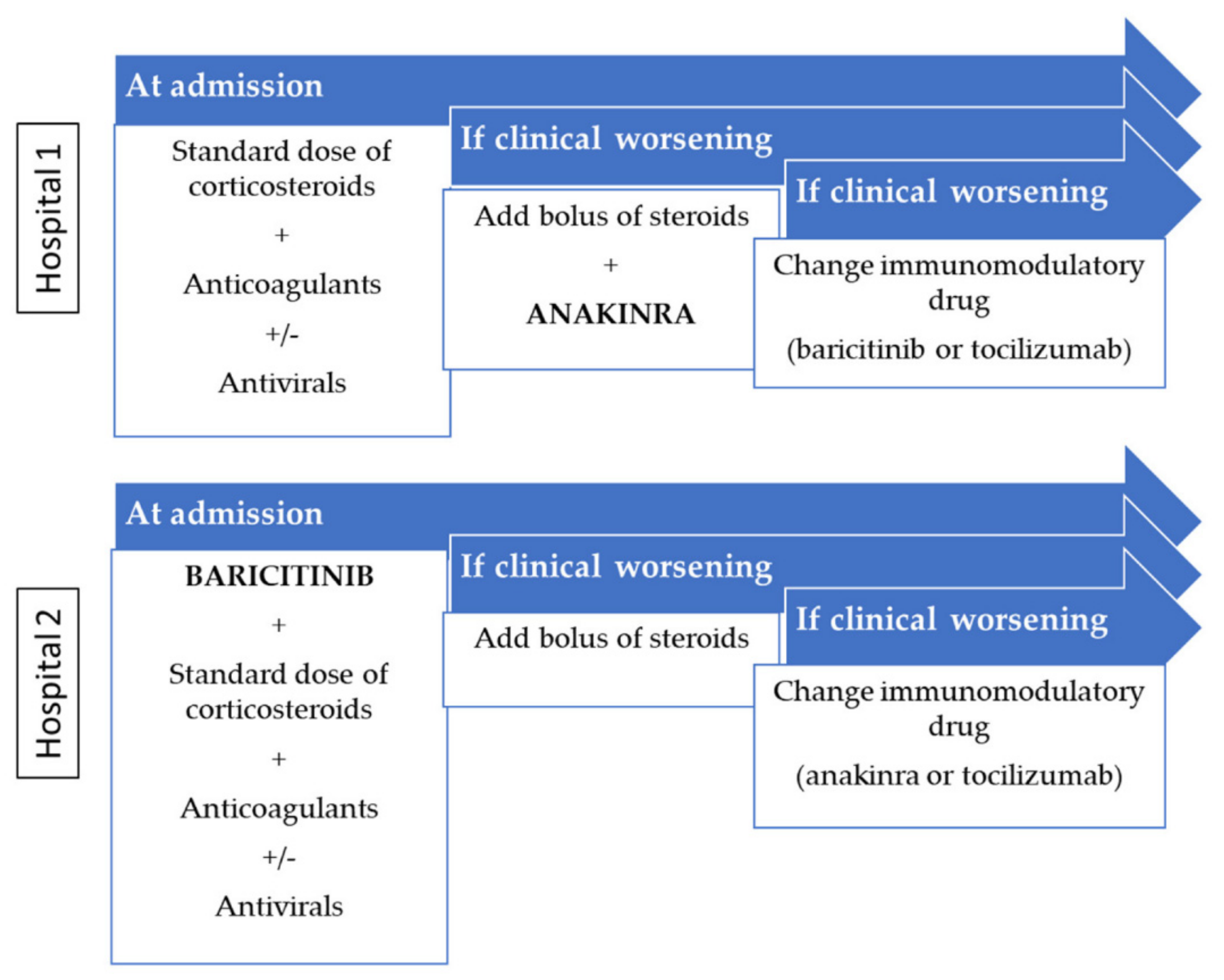
Jcm Free Full Text Anakinra Versus Baricitinib Different Strategies For Patients Hospitalized With Covid 19
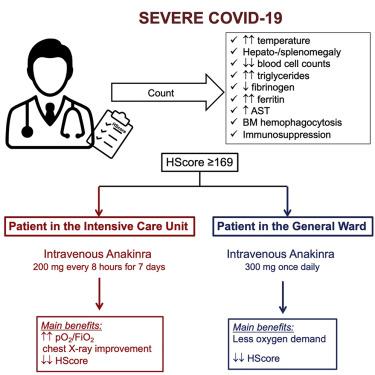
Favorable Anakinra Responses In Severe Covid 19 Patients With Secondary Hemophagocytic Lymphohistiocytosis Cell Host Microbe X Mol
Plos One Glucocorticoids With Low Dose Anti Il1 Anakinra Rescue In Severe Non Icu Covid 19 Infection A Cohort Study

Pulmcrit High Dose Anakinra For Covid 19 The Anti Inflammatory Trials Begin

Early Identification Of Covid 19 Cytokine Storm And Treatment With Anakinra Or Tocilizumab International Journal Of Infectious Diseases

Interleukin 1 Receptor Antagonist Anakinra In Association With Remdesivir In Severe Covid 19 A Case Report International Journal Of Infectious Diseases

Greek Study Suggests Mortality Benefit For Kineret In Covid 19 2021 05 20 Bioworld
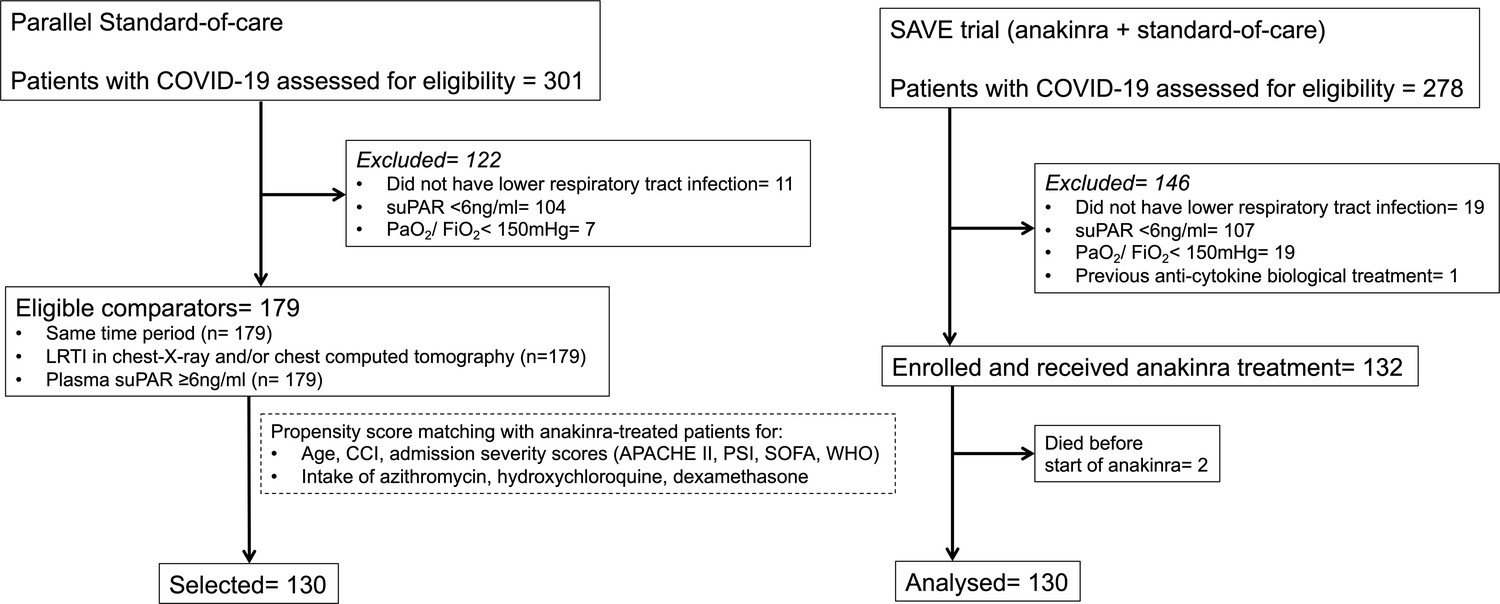
Figures And Data In An Open Label Trial Of Anakinra To Prevent Respiratory Failure In Covid 19 Elife

Kineret Improves Clinical Outcomes In Patients With Covid 19 Pneumonia
Anakinra Might Help Coronavirus Patients With Severe Respiratory Distress

Safety Perspectives On Presently Considered Drugs For The Treatment Of Covid 19 Penman 2020 British Journal Of Pharmacology Wiley Online Library

Covid 19 And Immunomodulator Immunosuppressant Use In Dermatology Journal Of The American Academy Of Dermatology

Il 1 Receptor Antagonist Anakinra In The Treatment Of Covid 19 Acute Respiratory Distress Syndrome A Retrospective Observational Study The Journal Of Immunology

Effectiveness Of Anakinra For Tocilizumab Refractory Severe Covid 19 A Single Centre Retrospective Comparative Study International Journal Of Infectious Diseases

The Pathogenesis And Treatment Of The Cytokine Storm In Covid 19 Journal Of Infection

Comparative Survival Analysis Of Immunomodulatory Therapy For Coronavirus Disease 2019 Cytokine Storm Chest

Anakinra In Hospitalized Patients With Severe Covid 19 Pneumonia Requiring Oxygen Therapy Results Of A Prospective Open Label Interventional Study International Journal Of Infectious Diseases
Biocentury A Mechanistic View Of Four Compounds That Disrupt Interleukin Pathways To Treat Severe Covid 19

